An eye drop potentially contaminated with fungus is being withdrawn from the market nationwide because it poses health risks to users.
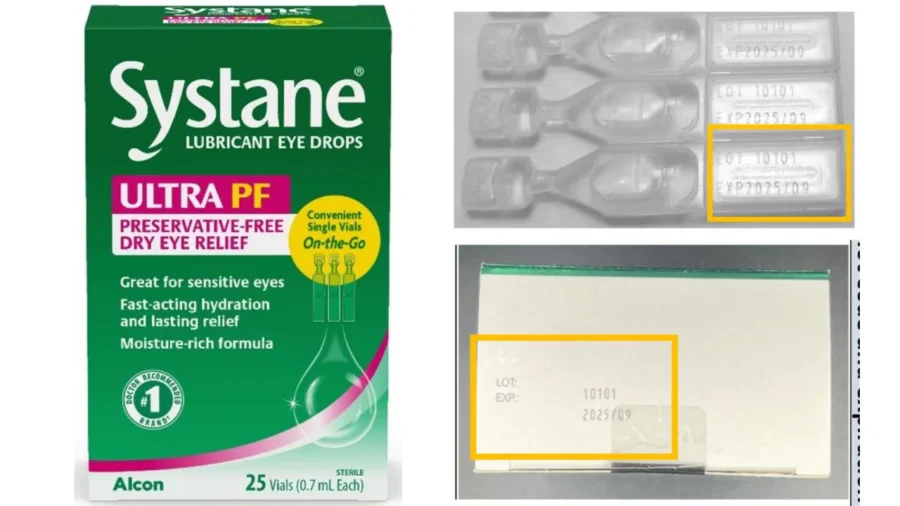
The recalled item is one lot of “Systane Lubricant Eye Drops Ultra PF, Single Vials On-the-Go” manufactured by Switzerland-based Alcon Laboratories, according to a Dec. 23 recall notice published by the U.S. Food and Drug Administration (FDA).
People who suffer from dry eye symptoms use the product to temporarily relieve irritation and burning sensations.
“Alcon evaluated a consumer complaint of foreign material observed inside a sealed single-use vial and determined the material to be fungal in nature,” the notice reads.
“Fungal contamination of an ophthalmic product is known to potentially cause eye infections,” it adds. “If an infection occurs, it may be vision-threatening, and in very rare cases potentially life-threatening in immunocompromised patients.”
The items were distributed through physical stores and sent out from internet purchases.
Alcon asked consumers who bought the recalled product to “stop using them immediately.” Consumers can return the item to the place of purchase and either get a replacement or a refund.
The company advised that consumers contact a physician if they experience any discomfort after using the item. Alcon said it hasn’t yet received any reports of people suffering from adverse effects from the recalled product.
Retailers and distributors with the products in stock were asked to discard them.
The product can be identified by its lot number 10101, pink and green carton design, a September 2025 expiration, with “ULTRA PF” and “Systane” mentioned on the carton. The package contains 25 vials. Alcon said it was notifying distributors and customers about the contaminated product.
Similar recalls linked to eye drop products have been made in recent years. In October 2023, Ohio-based Cardinal Health withdrew certain eye drops supplied by Velocity Pharma, citing the risk of eye infections.
In August 2023, Dr. Berne’s Whole Health Products, based in New Mexico, recalled its eye drop products due to fungal and bacterial contamination risks. The company received two adverse event reports linked to the products’ use.
The FDA said products such as eye drops present a “heightened risk of harm” since they circumvent some of the body’s natural defenses. Hence, sterility is a crucial aspect to consider when using eye drops.
Some ingredients, such as silver sulfate or methylsulfonylmethane, are inappropriate for use in eye drop products, according to the FDA, which suggests consumers check the label to ensure the item does not contain these ingredients.
“Wash your hands with soap and water before using eye drops, and do not touch the tip of the eye drop bottle to your hands, your eyes, your clothing, or any other surface to avoid contamination,” the agency said.
“Stop using eye drops if you experience any issue while using the products, such as discharge from the eye, pain, changes in vision or discomfort.”
Nasal Solution Recall
On Dec. 20, Pennsylvania-based Endo Inc. announced the voluntary recall of several lots of Adrenalin Chloride Solution (EPINEPHrine nasal solution).
The items were sold in 30-milliliter vials and distributed across the country to wholesale entities between Oct. 10, 2023, and Dec. 11, 2024. Their expiration dates ranged from January 2025 to March 2026.
“This product, which pre-dates the 1938 Federal Food, Drug & Cosmetic Act, was never submitted for approval by the FDA, and as such, is an unapproved drug for which safety and efficacy have not been established and, therefore, subject to recall,” the notice reads.
The FDA also determined that the product’s misleading label appeared similar to that of another product manufactured by the same company, Adrenalin (epinephrine injection), which has already received the agency’s approval.
The similar labels make it challenging to distinguish between the two items, “which can lead to potential administration errors,” according to the notice, which also warns that using the recalled product can lead to some severe health outcomes.
“There is a high probability that intravenous administration of the nasal product will result in patients receiving the wrong dose of epinephrine in emergency situations for serious, life-threatening conditions such as the treatment of anaphylaxis, blood pressure support, and cardiac arrest,” the notice reads.
In September, California-based Green Pharmaceuticals Inc. recalled its SnoreStop Nasal Spray, citing potential microbial contamination.
The items were marketed for use among individuals aged 5 and above. The FDA said the product made “unproven claims” that it would open the air passage and relieve users from nasal congestion.
From The Epoch Times